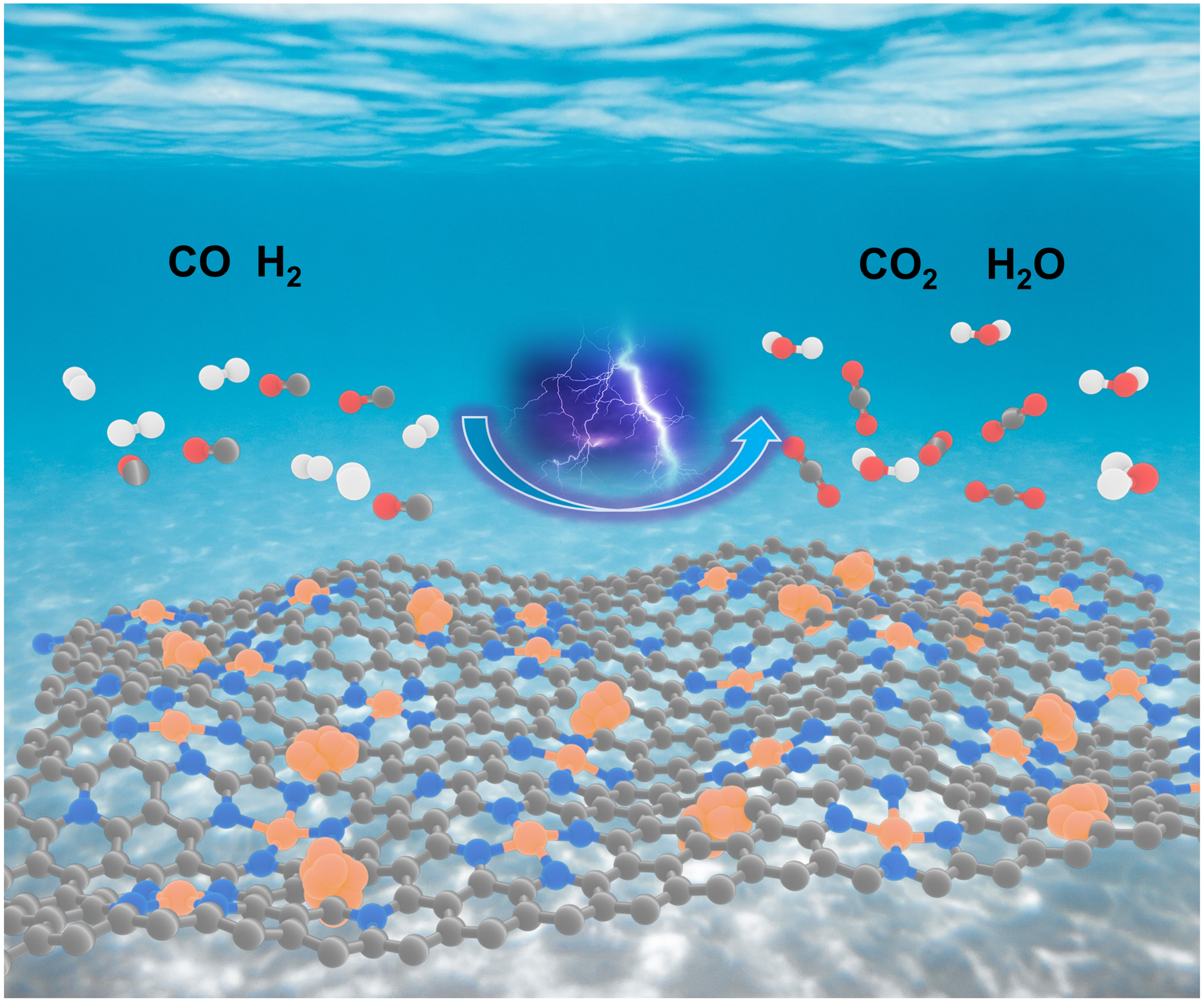
Hydrogen energy is regarded as the most promising clean energy in the 21st century, and proton exchange membrane fuel cell (PEMFC) is the key technology to build a hydrogen energy society. At present, the bottleneck restricting its development lies in the high cost of hydrogen production, storage, and transportation. An effective method to solve the above problems is to store hydrogen in liquid fuel with high hydrogen mass content, such as methanol, and release hydrogen in situ through liquid-phase reforming reaction when necessary. The generated hydrogen then enters the PEMFCs anode as fuel, so as to realize the effective series connection of the two devices. However, there is a loophole in this strategy because the hydrogen produced by small organic molecule reformation generally contains a certain amount of CO (~1000 ppm), while trace level CO (< 10 ppm) can seriously poison the Pt based catalysts used in PEMFCs anode. Therefore, the development of anode catalysts with high anti-poisoning ability is the key point.
Herein, we report an Ir-SACs&NPs/NC catalyst that contains both single-atom Ir sites and Ir nanoparticles, showing a prominent level of CO tolerance. Ir nanoparticles provide superb hydrogen oxidation (HOR) activity, and the atomically dispersed Ir sites exhibit excellent catalytic activity towards CO electro-oxidation (COOR) with an ultra-low onset oxidation potential of nearly 0 mV vs. RHE. According to pre-poisoning experiment, we find that the single atom sites are not only fast CO oxidizing catalysts themselves, but also act as efficient CO scavenger for approximated metal particles.